Page 137 - Physics
P. 137
Mechanical properties of matter
Mechanism of capillarity The diametre of the tube or opening
Capillarity occurs because The height of the liquid column is inversely proportional
of the intermolecular forces to the diametre of the tube or opening. The weight
between the liquid and the of the liquid column is proportional to the square of
surrounding solid surfaces. If the tube’s radius. It follows that a narrower tube will
the diametre of the opening draw a longer liquid column than a wider tube. For
is sufficiently small, the example, the water in the glass capillary tube will rise
combination of surface tension to a height approximated by 0.3 where d is the diametre
and adhesive forces between d
the liquid and the container (in centimetre) of the tube. Thus, if a glass tube has a
act to propel the liquid through diametre of 0.5 mm, water will rise to a height given
the opening. Note that, surface as; h = 0.3 cm = 6 cm.
tension is due to cohesive 0.05
forces. That is, capillarity is Tubes of different diameters will result to water columns
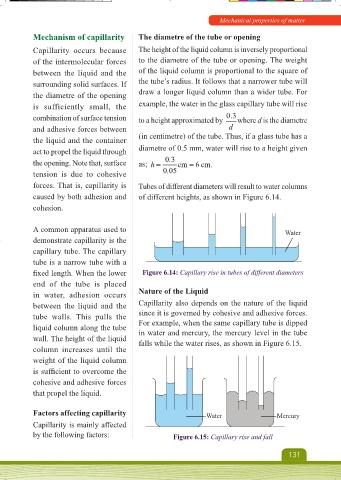
caused by both adhesion and of different heights, as shown in Figure 6.14.
cohesion.
A common apparatus used to Water
demonstrate capillarity is the
capillary tube. The capillary
tube is a narrow tube with a
fi xed length. When the lower Figure 6.14: Capillary rise in tubes of different diameters
end of the tube is placed
in water, adhesion occurs Nature of the Liquid
between the liquid and the Capillarity also depends on the nature of the liquid
tube walls. This pulls the since it is governed by cohesive and adhesive forces.
liquid column along the tube For example, when the same capillary tube is dipped
in water and mercury, the mercury level in the tube
wall. The height of the liquid falls while the water rises, as shown in Figure 6.15.
column increases until the
weight of the liquid column
is suffi cient to overcome the
cohesive and adhesive forces
that propel the liquid.
Factors affecting capillarity Water Mercury
Capillarity is mainly affected
by the following factors: Figure 6.15: Capillary rise and fall
131
Physics Form 1 Final.indd 131 16/10/2024 20:57