Page 44 - Biology_F5
P. 44
are alpha (α) and beta (β) isomers. The α form is when the hydroxyl (OH) group on carbon
atom number 1 projects below the ring while β form is when the OH group on carbon atom
number 1projects above the ring. Pentose sugars may form the five membered ring when
their carbon atom number 1 joins with the oxygen atom of carbon number 4, an example is as
shown in ribose and deoxyribose sugars (Figure 1.21 a and b). The only difference that exists
between ribose and deoxyribose sugar is that deoxyribose sugar lacks oxygen in carbon
number 2. Hexoses can form both six and five membered ring. For example glucose can exist
in two isomers of six membered rings (α- glucose and β-glucose). The formation of ring form
is when the oxygen atom of carbon number 5 joins to the carbon number 1 bearing the
aldehyde and transfer its hydrogen to it and break the bond to form the OH either above or
below the ring. The oxygen is part of the ring and carbon number 6 sticks up out of the ring.
The same process occurs in galactose. The only difference between galactose and glucose is
the different orientation of their hydroxyl group in carbon number 4 (Figure 1.21 c and d). In
fructose, the carbonyl group is in carbon number 2, so the ring is formed by the oxygen atom
of carbon number 5 joining with carbon number 2, leading to the formation of furan ring.
When the OH in carbon atom number 1 is projected below the ring, it becomes α-fructose
while when OH in carbon atom number 2 is projected above the ring is β-fructose (Figure
1.21 e). Fructose can also form the pyronose as in glucose
(a)
(b)
polysaccharides. The ring form occurs in aqueous solution and can form two isomers which
Figure 1.21 Open chain and ring form of (a) ribose and (b) deoxyribose
Cytology
Disaccharides (double sugars)
They are formed through condensation
of two monosaccharides; Examples of
disacharides include sucrose, maltose,
and lactose. They are composed of two
monosaccharide units bound together by
FOR ONLINE READING ONLY
a covalent bond known as a glycosidic
bond. They are formed via dehydration
(condensation) reaction resulting into
the loss of a hydrogen atom from
one monosaccharide and a hydroxyl
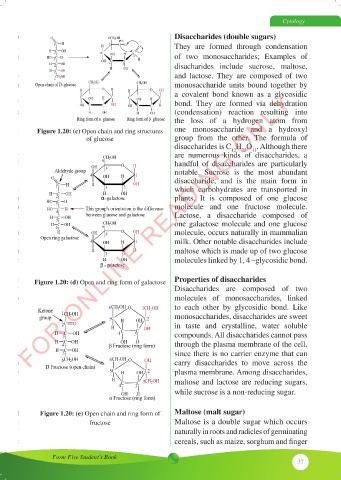
Figure 1.20: (c) Open chain and ring structures
Figure 1.21 (c) Open chain and ring structures of glucose
group from the other. The formula of
of glucose
Figure 1.21 (c) Open chain and ring structures of glucose disaccharides is C H O . Although there
are numerous kinds of disaccharides, a
12
22
11
handful of disaccharides are particularly
notable. Sucrose is the most abundant
disaccharide, and is the main form in
which carbohydrates are transported in
plants. It is composed of one glucose
molecule and one fructose molecule.
Lactose, a disaccharide composed of
one galactose molecule and one glucose
molecule, occurs naturally in mammalian
milk. Other notable disaccharides include
maltose which is made up of two glucose
molecules linked by 1, 4 –glycosidic bond.
Properties of disaccharides
Figure 1.20: (d) Open and ring form of galactose
Figure 1.21 (d) Open and ring form of galactose
Figure 1.21 (d) Open and ring form of galactose Disaccharides are composed of two
molecules of monosaccharides, linked
to each other by glycosidic bond. Like
monosaccharides, disaccharides are sweet
in taste and crystalline, water soluble
compounds. All disaccharides cannot pass
through the plasma membrane of the cell,
since there is no carrier enzyme that can
carry disaccharides to move across the
plasma membrane. Among disaccharides,
maltose and lactose are reducing sugars,
while sucrose is a non-reducing sugar.
Figure 1.21 (e) Open chain and ring form of fructose Maltose (malt sugar)
Figure 1.20: (e) Open chain and ring form of
Figure 1.21 (e) Open chain and ring form of fructose Maltose is a double sugar which occurs
fructose
naturally in roots and radicles of germinating
cereals, such as maize, sorghum and finger
Maltose (malt sugar)
Maltose (malt sugar) Form Five Student’s Book 37
Maltose is a double sugar which occurs naturally in roots and radicles of germinating cereals,
Maltose is a double sugar which occurs naturally in roots and radicles of germinating cereals,
such as maize, sorghum and finger millet. Artificially, it is made up by chemical combination
such as maize, sorghum and finger millet. Artificially, it is made up by chemical combination
of two a-glucose units. During this combination -OH group at carbons 1 and 4 of the two
of two a-glucose units. During this combination -OH group at carbons 1 and 4 of the two
glucose residues, are involved in formation of oxygen covalent bond called glycosidic bond.
glucose residues, are involved in formation of oxygen covalent bond called glycosidic bond.
Since it is formed between carbons 1 and 4, then it is termed a 1, 4-glycosidic bond. This
Since it is formed between carbons 1 and 4, then it is termed a 1, 4-glycosidic bond. This
process involves condensation, therefore, a molecule of water is lost (Figure 1.22). Maltose is
process involves condensation, therefore, a molecule of water is lost (Figure 1.22). Maltose is
a reducing sugar since it has a free aldehyde group in its molecule. In one of the glucose
a reducing sugar since it has a free aldehyde group in its molecule. In one of the glucose
units, the aldehyde at carbon 1 has been used in the formation of the bond, while in the
units, the aldehyde at carbon 1 has been used in the formation of the bond, while in the