Page 45 - Biology_F5
P. 45
Biology for Advanced Level Secondary Schools
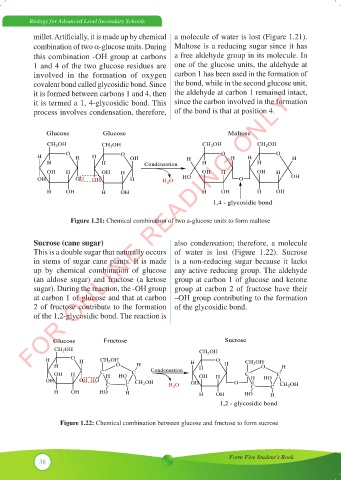
millet. Artificially, it is made up by chemical a molecule of water is lost (Figure 1.21).
combination of two a-glucose units. During Maltose is a reducing sugar since it has
this combination -OH group at carbons a free aldehyde group in its molecule. In
1 and 4 of the two glucose residues are one of the glucose units, the aldehyde at
involved in the formation of oxygen carbon 1 has been used in the formation of
covalent bond called glycosidic bond. Since the bond, while in the second glucose unit,
FOR ONLINE READING ONLY
it is formed between carbons 1 and 4, then the aldehyde at carbon 1 remained intact,
it is termed a 1, 4-glycosidic bond. This since the carbon involved in the formation
second glucose unit, the aldehyde at carbon 1 remained intact, since the carbon involved in
process involves condensation, therefore, of the bond is that at position 4.
the formation of the bond is that at position 4.
second glucose unit, the aldehyde at carbon 1 remained intact, since the carbon involved in
the formation of the bond is that at position 4.
Figure 1.22 Chemical combination of two a-glucose units to form maltose
Figure 1.21: Chemical combination of two a-glucose units to form maltose
Sucrose (cane sugar)
This is a double sugar that naturally occurs in stems of sugar cane plants. It is made up by
also condensation; therefore, a molecule
Sucrose (cane sugar)
Figure 1.22 Chemical combination of two a-glucose units to form maltose
chemical combination of glucose (an aldose sugar) and fructose (a ketose sugar). During the
This is a double sugar that naturally occurs of water is lost (Figure 1.22). Sucrose
reaction, the –OH group at carbon 1 of glucose and that at carbon 2 of fructose contribute to the
in stems of sugar cane plants. It is made is a non-reducing sugar because it lacks
Sucrose (cane sugar)
formation of the 1,2-glycosidic bond. The reaction is also condensation; therefore, a molecule
up by chemical combination of glucose any active reducing group. The aldehyde
This is a double sugar that naturally occurs in stems of sugar cane plants. It is made up by
of water is lost (Figure 1.23). Sucrose is a non-reducing sugar because it lacks any active
(an aldose sugar) and fructose (a ketose group at carbon 1 of glucose and ketone
chemical combination of glucose (an aldose sugar) and fructose (a ketose sugar). During the
reducing group. The aldehyde group at carbon 1 of glucose and ketone group at carbon 2 of
sugar). During the reaction, the -OH group group at carbon 2 of fructose have their
reaction, the –OH group at carbon 1 of glucose and that at carbon 2 of fructose contribute to the
fructose have their –OH group contributing to the formation of the glycosidic bond.
formation of the 1,2-glycosidic bond. The reaction is also condensation; therefore, a molecule
at carbon 1 of glucose and that at carbon –OH group contributing to the formation
2 of fructose contribute to the formation of the glycosidic bond.
of water is lost (Figure 1.23). Sucrose is a non-reducing sugar because it lacks any active
reducing group. The aldehyde group at carbon 1 of glucose and ketone group at carbon 2 of
of the 1,2-glycosidic bond. The reaction is
fructose have their –OH group contributing to the formation of the glycosidic bond.
Figure 1.23 Chemical combination between glucose and fructose to form sucrose
Lactose (milk sugar)
Figure 1.23 Chemical combination between glucose and fructose to form sucrose
Figure 1.22: Chemical combination between glucose and fructose to form sucrose
This is found exclusively in the milk of mammals and in milk products. Lactose is the only
carbohydrate of milk which is synthesized by mammary gland during lactation. It is derived
Lactose (milk sugar)
from the condensation of galactose and glucose linked by 1, 4 - glycosidic bond (Figure
This is found exclusively in the milk of mammals and in milk products. Lactose is the only
1.24).
carbohydrate of milk which is synthesized by mammary gland during lactation. It is derived
Form Five Student’s Book
from the condensation of galactose and glucose linked by 1, 4 - glycosidic bond (Figure
38
1.24).