Page 50 - Biology_F5
P. 50
Cytology
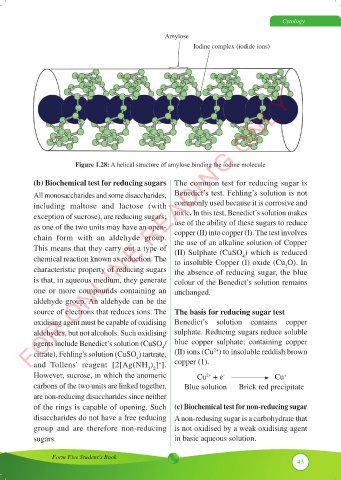
Amylose
Iodine complex (iodide ions)
FOR ONLINE READING ONLY
Figure 1.28: A helical structure of amylose binding the iodine molecule
(b) Biochemical test for reducing sugars The common test for reducing sugar is
All monosaccharides and some disaccharides, Benedict’s test. Fehling’s solution is not
including maltose and lactose (with commonly used because it is corrosive and
exception of sucrose), are reducing sugars; toxic. In this test, Benedict’s solution makes
as one of the two units may have an open- use of the ability of these sugars to reduce
copper (II) into copper (I). The test involves
chain form with an aldehyde group. the use of an alkaline solution of Copper
This means that they carry out a type of (II) Sulphate (CuSO ) which is reduced
4
chemical reaction known as reduction. The to insoluble Copper (I) oxide (Cu O). In
2
characteristic property of reducing sugars the absence of reducing sugar, the blue
is that, in aqueous medium, they generate colour of the Benedict’s solution remains
one or more compounds containing an unchanged.
aldehyde group. An aldehyde can be the
source of electrons that reduces ions. The The basis for reducing sugar test
oxidising agent must be capable of oxidising Benedict’s solution contains copper
aldehydes, but not alcohols. Such oxidising sulphate. Reducing sugars reduce soluble
agents include Benedict’s solution (CuSO / blue copper sulphate; containing copper
4
2+
citrate), Fehling's solution (CuSO ) tartrate, (II) ions (Cu ) to insoluble reddish brown
4
and Tollens' reagent [2[Ag(NH ) ] ]. copper (1).
+
3 2
However, sucrose, in which the anomeric Cu + e Cu +
-
2+
carbons of the two units are linked together, Blue solution Brick red precipitate
are non-reducing disaccharides since neither
of the rings is capable of opening. Such (c) Biochemical test for non-reducing sugar
disaccharides do not have a free reducing A non-redusing sugar is a carbohydrate that
group and are therefore non-reducing is not oxidised by a weak oxidising agent
sugars. in basic aqueous solution.
Form Five Student’s Book
43