Page 64 - Biology_F5
P. 64
Cytology
electrostatic interactions. Hydrogen bonds covalent bonds also contribute to the
are formed when hydrogen atom is shared formation of the tertiary structure.
with two other atoms. Hydrophobic Examples of tertiary structure of proteins
interactions, disulphide linkages and are enzymes and antibodies.
FOR ONLINE READING ONLY
-
+ -
-
- -
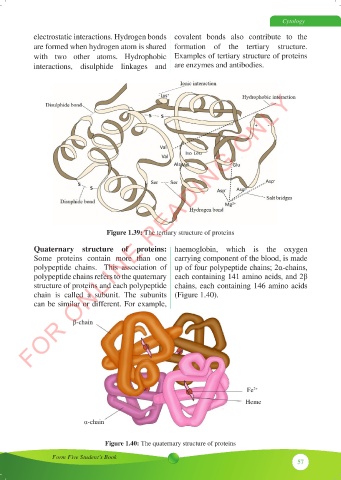
Figure 1.39: The tertiary structure of proteins
Quaternary structure of proteins: haemoglobin, which is the oxygen
Some proteins contain more than one carrying component of the blood, is made
polypeptide chains. This association of up of four polypeptide chains; 2α-chains,
polypeptide chains refers to the quaternary each containing 141 amino acids, and 2β
structure of proteins and each polypeptide chains, each containing 146 amino acids
chain is called a subunit. The subunits (Figure 1.40).
can be similar or different. For example,
β-chain
Fe 2+
Heme
α-chain
Figure 1.40: The quaternary structure of proteins
Form Five Student’s Book
57