Page 306 - Biology_F5
P. 306
Gas exchange and respiration
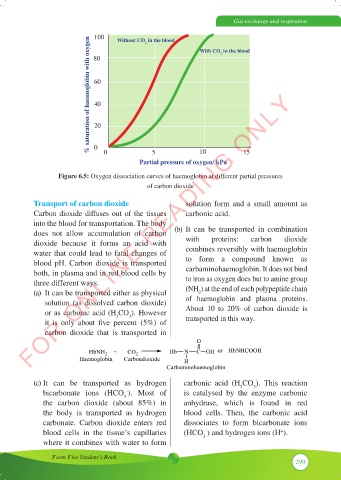
100
Without CO in the blood
% saturation of haemoglobin with oxygen
2
With CO in the blood
2
80
60
FOR ONLINE READING ONLY
40
20
0
0 5 10 15
Partial pressure of oxygen/ kPa
Figure 6.5: Oxygen dissociation curves of haemoglobin at different partial pressures
of carbon dioxide
Transport of carbon dioxide solution form and a small amount as
Carbon dioxide diffuses out of the tissues carbonic acid.
into the blood for transportation. The body
does not allow accumulation of carbon (b) It can be transported in combination
dioxide because it forms an acid with with proteins: carbon dioxide
water that could lead to fatal changes of combines reversibly with haemoglobin
blood pH. Carbon dioxide is transported to form a compound known as
both, in plasma and in red blood cells by carbaminohaemoglobin. It does not bind
three different ways. to iron as oxygen does but to amine group
(a) It can be transported either as physical (NH ) at the end of each polypeptide chain
2
solution (as dissolved carbon dioxide) of haemoglobin and plasma proteins.
or as carbonic acid (H CO ). However About 10 to 20% of carbon dioxide is
2
3
it is only about five percent (5%) of transported in this way.
carbon dioxide that is transported in
(c) It can be transported as hydrogen carbonic acid (H CO ). This reaction
2
3
bicarbonate ions (HCO ). Most of is catalysed by the enzyme carbonic
-
3
the carbon dioxide (about 85%) in anhydrase, which is found in red
the body is transported as hydrogen blood cells. Then, the carbonic acid
carbonate. Carbon dioxide enters red dissociates to form bicarbonate ions
blood cells in the tissue’s capillaries (HCO ) and hydrogen ions (H ).
+
-
3
where it combines with water to form
Form Five Student’s Book
299