Page 307 - Biology_F5
P. 307
Biology for Advanced Level Secondary Schools
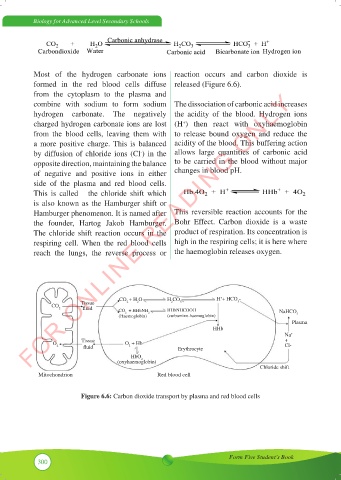
Most of the hydrogen carbonate ions reaction occurs and carbon dioxide is
formed in the red blood cells diffuse released (Figure 6.6).
FOR ONLINE READING ONLY
from the cytoplasm to the plasma and
combine with sodium to form sodium The dissociation of carbonic acid increases
hydrogen carbonate. The negatively the acidity of the blood. Hydrogen ions
charged hydrogen carbonate ions are lost (H ) then react with oxyhaemoglobin
+
from the blood cells, leaving them with to release bound oxygen and reduce the
a more positive charge. This is balanced acidity of the blood. This buffering action
by diffusion of chloride ions (Cl ) in the allows large quantities of carbonic acid
-
opposite direction, maintaining the balance to be carried in the blood without major
of negative and positive ions in either changes in blood pH.
side of the plasma and red blood cells.
This is called the chloride shift which
is also known as the Hamburger shift or
Hamburger phenomenon. It is named after This reversible reaction accounts for the
the founder, Hartog Jakob Hamburger. Bohr Effect. Carbon dioxide is a waste
The chloride shift reaction occurs in the product of respiration. Its concentration is
respiring cell. When the red blood cells high in the respiring cells; it is here where
reach the lungs, the reverse process or the haemoglobin releases oxygen.
CO + H O H CO H + HCO -
+
Tissue 2 2 2 3 3
CO 2 fluid CO + HHbNH HHbNHCOOH NaHCO
2
(Haemoglobin) 2 (carbamino-haemoglobin) 3
Plasma
HHb Na
+
O Tissue O + Hb +
2 fluid 2 Erythrocyte Cl-
HbO
2
(oxyhaemoglobin)
Chloride shift
Mitochondrion Red blood cell
Figure 6.6: Carbon dioxide transport by plasma and red blood cells
Form Five Student’s Book
300