Page 75 - Biology_F5
P. 75
Biology for Advanced Level Secondary Schools
changes in pH can cause enzymes to be as prosthetic groups. Only apoenzyme and
denatured and permanently lose their cofactor together are active as a catalyst.
functions. Enzymes in different locations The cofactor is either inorganic ions, usually
have different optimum pH values, since metal ions such as Fe , Mg , Cu , Cl
2+
2+
2+
-
their environmental conditions may or small organic molecules such as haem,
differ. For example, the enzyme “pepsin” biotin, Flavine adenine dinucleotide (FAD),
FOR ONLINE READING ONLY
functions best at pH value of around and Nicotineamide adenine dinucleotide
2 and is found in the stomach, which (NAD). They may also be vitamins called
contains hydrochloric acid. “Carbonic coenzymes. Some enzymes need metal ions
anhydrase”, which is a key enzyme in all and a coenzyme to become active.
living organisms works best at pH value
of around 7, and “chymotrypsin”, which Metal ion and/or coenzyme
is found in small intestine works best at pH
value of around 9.
Apoenzyme + Cofactor = Active enzyme
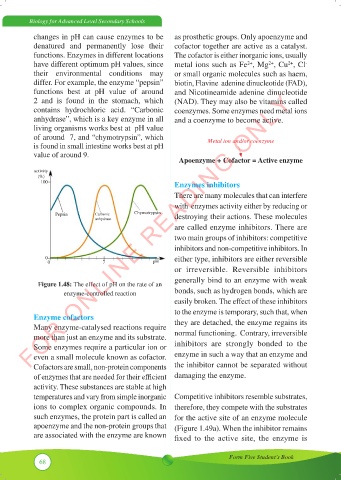
activity
(%)
100 Enzymes inhibitors
There are many molecules that can interfere
with enzymes activity either by reducing or
Pepsin Carbonic Chymotrypsin destroying their actions. These molecules
anhydrase
are called enzyme inhibitors. There are
two main groups of inhibitors: competitive
inhibitors and non-competitive inhibitors. In
0 either type, inhibitors are either reversible
0 7 pH
or irreversible. Reversible inhibitors
generally bind to an enzyme with weak
Figure 1.48: The effect of pH on the rate of an
enzyme-controlled reaction bonds, such as hydrogen bonds, which are
easily broken. The effect of these inhibitors
to the enzyme is temporary, such that, when
Enzyme cofactors
Many enzyme-catalysed reactions require they are detached, the enzyme regains its
more than just an enzyme and its substrate. normal functioning. Contrary, irreversible
Some enzymes require a particular ion or inhibitors are strongly bonded to the
even a small molecule known as cofactor. enzyme in such a way that an enzyme and
Cofactors are small, non-protein components the inhibitor cannot be separated without
of enzymes that are needed for their efficient damaging the enzyme.
activity. These substances are stable at high
temperatures and vary from simple inorganic Competitive inhibitors resemble substrates,
ions to complex organic compounds. In therefore, they compete with the substrates
such enzymes, the protein part is called an for the active site of an enzyme molecule
apoenzyme and the non-protein groups that (Figure 1.49a). When the inhibitor remains
are associated with the enzyme are known fixed to the active site, the enzyme is
Form Five Student’s Book
68