Page 47 - Agriculture_Form_Three
P. 47
Agriculture for Secondary Schools
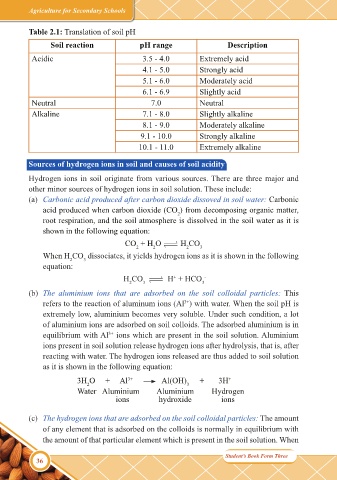
Table 2.1: Translation of soil pH
Soil reaction pH range Description
Acidic 3.5 - 4.0 Extremely acid
4.1 - 5.0 Strongly acid
5.1 - 6.0 Moderately acid
6.1 - 6.9 Slightly acid
Neutral 7.0 Neutral
Alkaline 7.1 - 8.0 Slightly alkaline
8.1 - 9.0 Moderately alkaline
9.1 - 10.0 Strongly alkaline
10.1 - 11.0 Extremely alkaline
Sources of hydrogen ions in soil and causes of soil acidity
Hydrogen ions in soil originate from various sources. There are three major and
other minor sources of hydrogen ions in soil solution. These include:
(a) Carbonic acid produced after carbon dioxide dissoved in soil water: Carbonic
acid produced when carbon dioxide (CO ) from decomposing organic matter,
2
root respiration, and the soil atmosphere is dissolved in the soil water as it is
shown in the following equation:
CO + H O ! ⇀!! H CO
↽ !!!
2 2 2 3
When H CO dissociates, it yields hydrogen ions as it is shown in the following
2
3
equation:
H CO ! ⇀!! H + HCO -
+
2 3 ↽ !!! 3
(b) The aluminium ions that are adsorbed on the soil colloidal particles: This
refers to the reaction of aluminum ions (Al ) with water. When the soil pH is
3+
extremely low, aluminium becomes very soluble. Under such condition, a lot
of aluminium ions are adsorbed on soil colloids. The adsorbed aluminium is in
equilibrium with Al ions which are present in the soil solution. Aluminium
3+
ions present in soil solution release hydrogen ions after hydrolysis, that is, after
reacting with water. The hydrogen ions released are thus added to soil solution
as it is shown in the following equation:
3H O + Al 3+ Al(OH) 3 + 3H +
2
Water Aluminium Aluminium Hydrogen
ions hydroxide ions
(c) The hydrogen ions that are adsorbed on the soil colloidal particles: The amount
of any element that is adsorbed on the colloids is normally in equilibrium with
the amount of that particular element which is present in the soil solution. When
Student’s Book Form Three
36
10/01/2025 12:31
AGRICULTURE FORM 3 9.11.2022.indd 36
AGRICULTURE FORM 3 9.11.2022.indd 36 10/01/2025 12:31