Page 46 - Agriculture_Form_Three
P. 46
Agriculture for Secondary Schools
The ability of soil to hold cation nutrients and exchange the ions in the adsorption
sites is called the Cation Exchange Capacity (CEC). The soil’s negatively charged
colloids can hold nutrients and prevent them from leaching beyond plant roots. The
greater cation exchange capacity the soil has, the more likely the soil will have a
higher fertility level. When combined with other measures of soil fertility, CEC is a
good indicator of soil fertility and productivity. Although both positive and negative
charges are present on colloid surfaces, most soils are dominated by negative
charges and have an overall negative charge. Therefore, more cations (positive ions)
are attracted to exchange sites than anions (negative ions). Thus, soils tend to have
greater CEC than Anion Exchange Capacities (AEC).
Fine-textured soils usually have a greater CEC than coarse soils. This is because
they have many clay colloids hence greater number of cation exchange sites to
which nutrients can adsorb. They are more fertile because they can provide more
nutrient elements to crop plants. In contrast, coarser or sandier soils have low CEC
due to small number of cation exchange sites to which plant nutrients can adsorb,
hence they are less fertile. Since organic matter also has negatively charged sites
that attract and hold cations, sandy soils rely on organic matter content to increase
cation exchange capacity. Thus, addition of organic matter to sandy soils improves
their fertility and productivity.
Soil pH
Soil pH refers to a soil’s acidity or alkalinity and is the measure of hydrogen ions
(H ) present in a soil solution. The main determinant of soil pH is the concentration
+
of hydrogen and hydroxyl ions in the soil solution. A high amount of H relates to a
+
low pH value (acidic) while OH relates with high pH (alkaline). Soil pH is normally
-
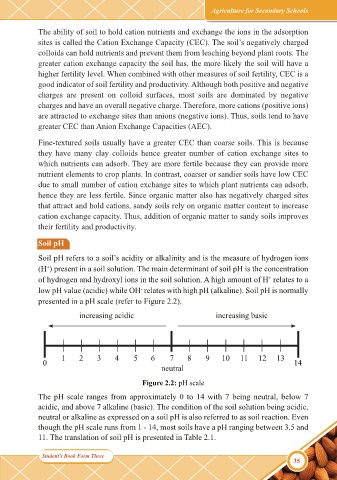
presented in a pH scale (refer to Figure 2.2).
increasing acidic increasing basic
1 2 3 4 5 6 7 8 9 10 11 12 13
0 14
neutral
Figure 2.2: pH scale
The pH scale ranges from approximately 0 to 14 with 7 being neutral, below 7
acidic, and above 7 alkaline (basic). The condition of the soil solution being acidic,
neutral or alkaline as expressed on a soil pH is also referred to as soil reaction. Even
though the pH scale runs from 1 - 14, most soils have a pH ranging between 3.5 and
11. The translation of soil pH is presented in Table 2.1.
Student’
Student’s Book Form Twos Book Form Three
35
10/01/2025 12:31
AGRICULTURE FORM 3 9.11.2022.indd 35 10/01/2025 12:31
AGRICULTURE FORM 3 9.11.2022.indd 35